Day 2 :
Keynote Forum
Mark Caulfield
William Harvey Research Institute & Queen Mary University of London, UK
Keynote: Advances in the genomics of blood pressure: Time for translation
Time : 09:05-09:40

Biography:
Professor Mark Caulfield graduated in medicine in 1984 and trained in Clinical Pharmacology at St Bartholomew’s Hospital where he developed a major programme in genetics of blood pressure regulation. In 2002 he became Co-Director of the William Harvey Research Institute at Queen Mary University of London which he grew from 140 to 530 clinicians and scientists and a major worldwide pharmacological centre focused on cardiovascular, inflammation and endocrine research. He was President of the British Hypertension Society and served on the council of the European Society of Hypertension. In 2013 he became an NIHR Senior Investigator and Chief Scientist for the 100,000 Genomes Project.
Abstract:
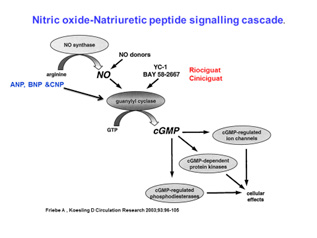
Statement of the problem: Hypertension is the commonest cardiovascular worldwide with an anticipated 1.5 billion people with high blood pressure by 2025. It arises from a complex interplay between genes and lifestyle. From family studies between 30-50% of the heritability of blood pressure is due genetic influences or gene plus lifestyle. Approximately 8-12% of hypertensives cannot tolerate or are resistant to current therapies. Understanding the genes underpinning blood pressure could identify new biological pathways for innovative therapeutics. In genome wide studies of blood pressure now expanded to more than 1000 gene loci for blood pressure discovered and validated in over 1 million people. Many of these loci identify new biological pathways and some repurposing opportunities for existing therapies used for other disorders. A genetic risk score of all aggregate variants at 1000 loci suggested that in the over 50 year olds these loci cause a potential 10 mm Hg rise in blood pressure. This prompts the question is it tie to translate these findings into the clinic. First a targeted gene chip could identify those at risk in early life and enable lifestyle measures such as exercise a diet rich in fruit and vegetables, maintenance of an ideal body weight and reduced alcohol intake. In addition in mechanistic studies we and others have identified potential therapies acting on the nitric oxide-natriuretic peptide pathway including beetroot juice and c-natriuretic peptide mimetics. We can also now deploy next generation sequencing techniques to diagnose the cause of rare syndromic forms of hypertension and the impact of that will be explored.
Recent Publications
1. Warren HR, Evangelou E, Cabrera CP, Gao H, Ren M, Mifsud B, and multiple co-authors then, Caulfield M., Elliott P. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017 Mar; 49(3):403-415. doi: 10.1038/ng.3768.
2. Georg B. Ehret then multiple co-authors then Mark J. Caulfield, Toby Johnson. Genetic variants from novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478(7367):103-9.
3. Louise V Wain, then multiple authors then Mark J Caulfield, Dabeeru C Rao, Martin D Tobin, Paul Elliott, Cornelia M van Duijn. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nature Genetics 2011 Sep 11; 43(10):1005-11.
4. Newton-Cheh C, then 152 co-authors then Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nature Genetics 2009 May 10. [Epub ahead of print] PubMed PMID: 19430483.
Keynote Forum
Rohit Arora
Professor of Medicine, Chicago Medical School, USA
Keynote: Effect of smoking on coronary microvasculature in hospitalized chest pain patients
Time : 09:40-10:15

Biography:
Dr. Rohit Arora is currently Professor of Medicine and Professor of Physiology and Biophysics, Chicago Medical School, Chicago, Illinois where he is also Chairman of Cardiology and Vice- Chairman of Medicine, Department of Medicine. He is also Chairman of Medicine and Chief of Cardiology at the Principal Teaching Hospital at the James Lovell FHCC, North Chicago IL. Previously, he was Chief of Cardiology at UMDNJ and Director of Interventional Cardiology and Catheterization Laboratory, New Jersey, and Director of Critical Cardiology, and Director of Cardiac Care Units at Columbia Presbyterian Medical Center, at Columbia University in New York. Dr. Arora’s residency training was at the Mount Sinai School of Medicine, New York, with a fellowship at Mount Sinai Medical Centre in New York, Nuclear cardiology fellowship also at Mount Sinai, Interventional fellowship at the Cleveland Clinic Foundation, Cleveland, Ohio. He does lectureship in thrombosis and vascular disease at Montefiore Medical Centre/Albert Einstein School of Medicine, NY. Dr Arora’s research interests include thrombosis, refractory angina, EECP, lipids, vascular biology of the endothelium, interventional, and preventive cardiology. He has authored or co-authored more than five hundred papers and abstracts, and is the recipient of numerous awards & honors. Dr Arora is on the editorial board of numerous medical journals, and was the editor of Heart International. He has co-authored a textbook on Clinical Autonomic Dysfunction, available on Amazon, by Springer. He is nominated to the FDA device panel for devise and radiological devices. He has performed pioneering studies in refractory angina and Enhanced External Counter-pulsation.
Abstract:
Background: Multiple studies demonstrate increased Thrombosis in Myocardial Infarction (TIMI) frame count (cTFC) among tobacco smokers (TS) even in the absence of epicardial coronary artery disease (CAD), suggestive of coronary microvascular dysfunction (MD). Among those patients who present with chest pain and a positive stress test, we hypothesize that the extent of MD is larger among TS when compared to non-smokers (NS).
Method: In this retrospective study, patients who underwent coronary angiogram for chest pain with a positive stress test were grouped into TS and NS. Among these, those who were free of a significant CAD, vasospasm or recent myocardial infarction were randomly chosen: 82 TS and 127 NS. Presence of coronary MD was assessed using cTFC and TIMI perfusion grade (TMPG).
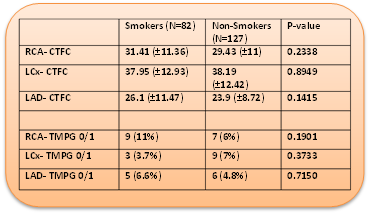
Results: At baseline, TS were significantly younger compared to NS (mean age in years 50.2 vs 58.56, p<0.0001) with significantly more females in the NS group. The cTFC and TMPG for left anterior descending, left circumflex and right coronary arteries though significantly higher in both groups, when compared to published normals, there was no significant difference observed. Additional sub-group analysis for gender and age (<50 years and ≥50 years) also did not demonstrate any significant differences. Furthermore, among patients with no history of hypertension, diabetes mellitus and hyperlipidemia, no significant differences were observed between the two groups.
Conclusion: Increased cTFC in both the groups indicates increased coronary microvascular resistance suggesting MD, which may be the reason for a positive stress test. This study demonstrates that among patients presenting with chest pain and a positive stress test, in the absence of significant epicardial CAD, tobacco smoking does not further increase MD.
Recent Publications:
- Crea F, Camici PG, Bairey Merz CN.Coronary microvascular dysfunction: an update.Eur Heart J. 2014 May;35(17):1101-11. doi: 10.1093/eurheartj/eht513.
- The TIMI Study Group. The Thrombolysis In Myocardial Infarction (TIMI) trial. N Engl J Med. 1985;31:932–936.
- Gibson CM, Cannon CP, Murphy SA, et al. Relationship of TIMI Myocardial Perfusion Grade to Mortality After Administration of Thrombolytic Drugs. Circulation. 2000;101:125.
- Gibson CM, Cannon CP, Daley WL, et al. TIMI Frame Count. Circulation. 1996;93:879-888.
- Erbay, Ali Riza et al. “Documentation of slow coronary flow by the thrombolysis in myocardial infarction frame count in habitual smokers with angiographically normal coronary arteries.” Heart and Vessels 19 (2004): 271-274.
Keynote Forum
Antonis A. Armoundas
Assistant Professor, Harvard Medical School, USA
Keynote: Real-time detection and suppression of repolarization alternans as antiarrhythmic therapy
Time : 09:40-10:15

Biography:
Antonis A Armoundas completed his BS in Electrical Engineering from National Technical University of Athens, Athens, Greece, in 1991 and MS in Biomedical Engineering from Boston University, Boston, MA, in 1994. He received PhD in Nuclear Engineering, from the Massachusetts Institute of Technology (MIT), in 1999. He was an American Heart Association sponsored Post-doctoral Fellow at the Division of Molecular Cardiobiology and the Department of Biomedical Engineering at Johns Hopkins University. Now, he is a National Institute of Health supported Principal Investigator at Massachusetts General Hospital and an Assistant Professor at Harvard Medical School, while he maintains an appointment at M.I.T. He has authored more than 80 high-impact peer-reviewed journal articles and book chapters, and he also holds six patents. His
research interests include “Biomedical signal processing, forward and inverse problem solutions, and cellular electrophysiology methods”.
Abstract:
Background: This study investigates the spatio-temporal variability of intracardiac repolarization alternans (RA) and its relationship to arrhythmia susceptibility in a swine acute myocardial ischemia (MI) model.
Methods & Results: We developed a real-time multi-channel repolarization signal acquisition, display and analysis system to record electrocardiographic signals from catheters in the right ventricle, coronary sinus and left ventricle prior to and following circumflex coronary artery balloon occlusion. We found that RA is detectable within 4 minutes following the onset ischemia, and is most prominently seen during the first half of the repolarization interval. We developed a novel, clinically-applicable intracardiac lead system based on a triangular arrangement of leads spanning the right ventricular (RV) and coronary sinus (CS) catheters which provided the highest sensitivity for intracardiac RA detection when compared to any other far-field bipolar sensing configurations (p < 0.0001). The magnitude of RA was used to adjust pacing stimuli delivered during the absolute refractory period (ARP) aimed to reduce RA. We found that the pacing pulse polarity and the phase polarity are sufficient parameters to suppress RA. To calibrate the pacing stimuli, we estimated the required charge to induce one μV [one unit] change in the alternans voltage [and Kscore] on CS and LV leads as 0.05 ± 0.025 [0.32 ± 0.29] and 0.06 ± 0.033 [0.33 ± 0.37] μC, respectively. Using this approach, we demonstrated the ability to suppress spontaneous RA following acute MI. Overall, pacing during the ARP resulted in a significant decrease in alternans
voltage and Kscore and reduced arrhythmia susceptibility (p<0.01).
Conclusion: RA can be reliably detected through a novel triangular RV-CS lead configuration. Electrical stimulation during the ARP can be used to suppress RA, in vivo. Our findings may have important implications in developing methods to prevent the onset of ventricular arrhythmias.
Recent Publications
1. Merchant FM and Armoundas A A. Role of substrate and triggers in the genesis of cardiac alternans, from the myocyte to the whole heart: Implications for therapy. Circulation. 2012;125:539-549.
2. Sayadi O, Puppala D, Ishaque N, Doddamani R, Merchant F M, Barrett C, Singh J P, Heist E K, Mela T, Martinez J P, Laguna P and Armoundas A A. A novel method to capture the onset of dynamic electrocardiographic ischemic changes and its implications to arrhythmia susceptibility. J Am Heart Assoc. 2014;3.
3. Sayadi O, Merchant F M, Puppala D, Mela T, Singh J P, Heist E K, Owen C and Armoundas A A. A novel method for determining the phase of t-wave alternans: Diagnostic and therapeutic implications. Circ Arrhythm Electrophysiol. 2013;6:818-826.
4. Merchant F M, Sayadi O, Moazzami K, Puppala D and Armoundas A A. T-wave alternans as an arrhythmic risk stratifier: State of the art. Curr Cardiol Rep. 2013;15:398.
5. Merchant F M, Sayadi O, Puppala D, Moazzami K, Heller V and Armoundas A A. A translational approach to probe the proarrhythmic potential of cardiac alternans: A reversible overture to arrhythmogenesis? Am J Physiol Heart Circ Physiol. 2014;306:H465-474.
